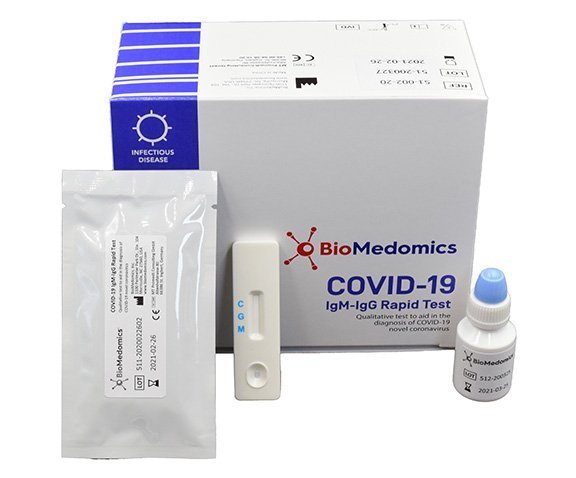
COVID-19 IgM/IgG Rapid Test
BioMedomics has developed and launched one of the world’s first rapid point-of-care lateral flow immunoassays to aid in the diagnosis of coronavirus infection. The test detects both early marker and late marker, IgM/IgG antibodies in human finger-prick (capillary) or venous whole blood, serum, and plasma samples.
There is a critical, global need for serology assays that can complement nucleic acid (PCR) tests for diagnosing COVID-19 infection. Although critically important, PCR tests are only positive during the brief window of acute infection, after which they become negative. And while serology tests are not as effective as PCR early in acute infection, they are able to detect COVID-19 antibodies for a prolonged period of time after disease resolution, which enables identification of prior infection. Knowledge of prior infection is epidemiologically important and represents a significant unmet need in the management of the COVID-19 pandemic.
Features & Benefits
BioMedomics Rapid IgM-IgG Combined Antibody Test for COVID-19 is used to qualitatively detect IgG and IgM antibodies of the novel coronavirus in human serum, plasma or whole blood in vitro.
Precise
- Works with whole blood, serum, & plasma
- Tests for both IgM and IgG antibodies
- Validated using PCR
Fast
- 10-15 minutes per test
- Intuitive visual interpretation
- No special equipment needed
How It Works

Steps
1. Collect blood/serum/plasma sample.
2. Add blood/serum/plasma sample to sample well.
3. Place 2-3 drops of buffer in sample well.
4. Read results after 10 minutes and no more than 15 minutes.
Results